In July 2016, the American Journal of Clinical Nutrition published a study that claimed to falsify or refute the “carbohydrate-insulin hypothesis” for obesity. This is a theory promoted by Dr. Jason Fung and others who criticize diets high in sugar and carbohydrates as promoting high insulin blood levels. The high blood insulin, in turn, suppresses the body’s ability to release and burn fat and instead drives fat into fat cells making a person fatter. Further claims include the idea that a person’s basic metabolic rate will slow in the presence of these elevated insulin levels (Hyperinsulinemia).
The study was funded by the Nutritional Science Initiative, an organization founded with the help of Gary Taubes, an advocate of the carbohydrate-insulin hypothesis. The lead scientist of the study, Dr. Kevin Hall, explains his work in this interview.
Obviously, Jason Fung doesn’t agree. He responds in Here’s $5, Kevin Hall, go buy yourself a clue.
Here is my simplified (perhaps oversimplified) explanation of what was done in the experiment:
- The experiment was done with 17 fat men. They were confined to a metabolic ward for two months where all of their food consumption and exercise were carefully controlled and measured.
- They were fed two diets. For the first month, they consumed a base diet (BD) where ~25% of total calories came from sugars. For the second month, they consumed ketogenic diet where <2% of total calories came from sugars.
- Actual energy expenditure was measured directly by having the subjects spend two days each week in an Energy Expenditure chamber. Details of how this was done are given in the paper.
- For the first two weeks on the base diet, the number of calories each person consumed was adjusted to achieve “energy balance”, meaning that they should be burning up as many calories as they eat. After two weeks, the number of calories each subject ate each day was “clamped” at that energy balance level and was kept the same for the next two weeks of the BD and for the four weeks of the KD diet.
- The subjects lost weight, including fat during the last two weeks of the BD diet when they were supposed to be in energy balance. This is called “surprising” in the study.
- Once the subjects switched to the KD diet, their weight dropped faster for a few days, then leveled off to a flat or much slower weight loss.
- During the first two weeks of the KD diet, the rate of reduction in body fat actually went down. For the last two weeks of the KD diet, the rate of reduction of body fat went back up to about the same rate as in the last two weeks of the BD.
- The subjects base energy consumption went up rapidly in the first week of the KD diet, then lowered from that higher level over the course of the three weeks. The net increase in base metabolism was barely significant according to the statistical analysis.
- The daily insulin secretion rapidly and persistently decreased by 47% after the introduction of the KD and total urinary ketone excretion increased >10-fold. These confirm that the patients were in ketosis on the KD diet.
My summary of the conclusions of the study is as follows. I use quotes from the article:
- The carbohydrate–insulin model predicts a greater rate of body fat loss during the KD period. Our data do not support this prediction.
- The carbohydrate–insulin model predicts that the KD would lead to increased EE, thereby resulting in a metabolic advantage amounting to ~300–600 kcal/d (21, 22). Our data do not support EE increases of that magnitude.
- Mathematical model simulations predicted that cutting dietary carbohydrates to very low amounts would reverse this trend [high carbohydrates causing a decrease in EE) and lead to slightly increased EE. This prediction appears to have been borne out in our data.
- In summary, we found that a carefully controlled isocaloric KD coincided with small increases in EE that waned over time. Despite rapid, substantial, and persistent reductions in daily insulin secretion and RQ after introducing the KD, we observed a slowing of body fat loss. Therefore, our data do not support the carbohydrate–insulin model predictions of physiologically relevant increases in EE or greater body fat loss in response to an isocaloric KD.
What should we think of this study? Here are Dr. Fung’s criticisms as I understand him.
First, Fung points out a weakness in the study. He says, “When patients embarked on their run in phase, they were switched to a 2700 calorie/day high sugar high carb diet, meant to replicate the Standard American Diet (SAD) . . . nobody believes it should cause fat loss. But it did. Why?”
Fung explains this as a common effect of this kind of experiment, “Anybody who has done research knows why. It’s the effect of going into a study and knowing that people are testing you. It’s a universal effect.”
To my surprise, the authors of the paper admit this is true! In the discussion portion, they say, “A major limitation of our study is the unintentional weight loss. Despite slight positive energy balance during the chamber days, the overall negative energy balance amounted to ~300 kcal/d and was likely due to greater spontaneous physical activity on non-chamber days. This occurred despite confining the subjects to metabolic wards and our best efforts to maintain constant activity levels by prescribing 90 min of fixed intensity stationary cycling exercise every day.” The experiment subjects exercised more at the start to perform better for the test because they knew they were being watched. The question is did this behavior persist during the last half of the experiment?
The paper implies that carbohydrate-insulin model prediction a greater rate of fat loss on a KD diet is false when they say their data does not support this prediction. But this conclusion is worthless if the initial fat loss on their BD is an artifact of the experiment.
Second, It also calls into question their conclusion that the carbohydrate-insulin model predicts that the KD would lead to increased EE, thereby resulting in a metabolic advantage amounting to ~300-600 calories per day. Fung points out that they did find an increase in EE of 57 calories per day that correlated with the KD with a 99.96% chance is not coincidental. Now, this 57 calorie difference is not great, but it appears to be absolute. When we speak of metabolic advantage, we have to ask compared to what? As I see it, no conclusion can be drawn regarding the magnitude of any metabolic advantage without an adequate measure of expected decreased in EE or basal metabolism from calorie-restricted diets.
And that is a gaping hole that I see in the study. The big criticism of calorie-restricted diets based on the calories-in-calories-out model concerns metabolic damage caused by these diets. Fung refers to a 2012 study spear-headed by the well known Havard nutritional researcher Dr. David Ludwig, Effects of dietary composition on energy expenditure during weight-loss maintenance.
That study compares the basal metabolic weight of subjects during the maintenance phase after wieght loss. Fung reproduced the following graphic:
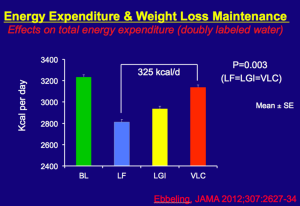
This summarizes the results of the Havard study. After dieting, the energy expenditure of the dieters was lowered. That is not surprising. Overweight people in general burn more calories to maintain body heat for their larger body mass, so overall energy expenditure is expected to decrease some after wieght loss. But notice the difference between those who lost the weight on a low fat (LF) diet versus those who lost the weight on a very low carbohydrate diet (VLC). The difference in 325 calories per day. That is where the metabolic advantage becomes evident. The reason for the advantage has more to do with the harm of calorie-restricted diets than it has to do with metabolic advantages conferred by a KD. It seems to me that this difference (this advantage) would persist even if we showed that weight maintenance on a KD did not alter EE at all.
A further issue which occurs to me concerns whether or not the obese subjects were insulin resistant. Obese people are in fact typically insulin resistant. Typically, insulin resistant people will maintain a much higher level of blood insulin for a longer time period following a meal. My question is how this might affect energy expenditure. It would be nice to see an experiment like this performed on people with normal weight and known not to be insulin resistant.

